Building biotechnologies
July 12, 2021
By Jinhong (Andrew) Kim

SNC-Lavalin provided EPCM services for Aprogen’s campus in Osong, South Korea. The project was completed in 2019. Images courtesy SNC-Lavalin.
Aprogen Biologics, a biotechnology company in South Korea, planned to use an advanced bioreactor technology to expand their biopharmaceutical production capacity at its campus in Osong. While planning the project, Aprogen selected SNC-Lavalin for EPCM services, due to having strong in-house expertise in biotechnology and global GMP regulations.
SNC-Lavalin’s Canadian team took a leadership role in engineering in collaboration with SNC-Lavalin’s Korean team and delivered the expansion project within time and budget, to allow Aprogen to fulfil local needs for autoimmune disease and cancer treatments and explore international needs.
Social and economic benefits
Aprogen Biologics is a biotechnology company with state-of-the-art technologies in protein and antibody engineering that allow for the efficient production of drug products to treat autoimmune diseases and various types of cancer.
Aprogen planned to expand their production capacity to fulfil local needs as well as explore North America’s needs for their new antibody-based treatments. This project covered the engineering and construction management for the biopharmaceutical manufacturing facility expansion, including two 2,000-L scale perfusion-based bioreactor lines and their purification process, filling and packaging facility, automated warehouse for ambient and cold storage, flammable storage buildings, and wastewater treatment facility expansion.
According to top trends in biopharmaceutical manufacturing, three major trends were implemented in this project to deliver a flexible and cost-effective facility with reduced capital investment and construction timelines: single-use systems, flexible facilities driven by modularity, and semi-continuous biomanufacturing.
As early as 2006, implementation of single use, sterile disposable systems in place of conventional reusable stainless-steel equipment began to impact facilities design and offer flexible solutions requiring less time-consuming, labour-intensive, expensive and energy-intensive cleaning and re-sterilization processes.
Standardization, rapid development, and reduced execution schedule are all current drivers to investigate modular delivery platforms for manufacturing assets that can be easily replicated in multiple locations. The standardized facility design developed in part by SNC-Lavalin for Aprogen’s existing facility could be readily adopted for this expansion project. Modular units also gave increased flexibility in operation and simplification of manufacturing itself. Commercial application of continuous biomanufacturing through high-yield perfusion cell culture processing is still in early stages.
SNC-Lavalin’s biopharmaceutical process engineers worked with Aprogen to scale-up their R&D perfusion process, allowing for smaller bioreactors (2,000 L) to be used to achieve double the product quantity possible with four 5,000 L bioreactors, which greatly reduced capital and operational costs.
The cutting-edge design concept implemented by SNC-Lavalin during project execution greatly benefits public health as it further improved Aprogen’s manufacturing efficiency for production of new cost-effective drug treatments to supply local and global markets and can allow Aprogen to sooner commercialize other new drug products in their pipeline.
This project was delivered with up-to-date technologies and new trends, some of which the industry is headed towards, and some of which were designed as “hybrid” solutions that are still fully supported by regulatory agencies including the FDA (U.S.), EMA (Europe), PMDA (Japan) and MFDS (Korea), and profoundly contribute to the public health and medical welfare.
Technology transfer
Process engineers from SNC-Lavalin’s Toronto and Montreal offices – having a wide depth of GMP experience based on FDA, EMEA and Health Canada regulations – collaborated closely with SNC-Lavalin’s local Korean engineers to deliver core biopharmaceutical manufacturing facility designs with technologies that both met the client’s requirements and incorporated current trends in GMP that the client is not experienced with. SNC-Lavalin introduced the latest technologies to the client in Korea while ensuring compliance with regulations in Korea, and various laws and regulations in construction.
The design of the automated warehouse for GMP product storage, designed as rack building type to handle more than 4,000 cells, was a critical component of the project due to the strict temperature distribution requirements for ensuring product quality. Although local suppliers have expertise in automatic warehouse technologies, SNC-Lavalin offered expertise in the application of these technologies for GMP purposes. The SNC-Lavalin Canadian team used CFD to perform extensive simulations of air flow distribution to finalize an acceptable mechanical design prior to construction, which was later verified through cold mapping.
During construction, SNC-Lavalin supported selection of various materials and construction methods to ensure compliance with current standards, and procurement for process equipment. Technical reviews of design, specification and fabrication from multiple suppliers was also supported. Together with Korean engineers, all of the discipline leaders from Canada were greatly involved in each project execution phase to support the necessary consultancy for construction and equipment installation, supporting Aprogen in their plan to supply drug treatments to a broad, global market.
Environmental benefits
For this project, vibration sensors and monitoring meters were designed and installed on pumps, motors and other various locations, such as in motors for air handling units, so that real-time data can be collected, monitored and analyzed for potential application of next generation maintenance approaches such as predictive maintenance. This is cutting-edge technology that brings the client closer towards Industry 4.0 (or Pharma 4.0). The accumulated data can be analyzed to maximize the usage of each facility asset. Previous systems in the pharmaceutical industry only maintenance the facility asset based on a set usage time.
Biopharmaceutical manufacturing facilities traditionally use a significant amount of water for production, such as for buffer preparation and cleaning of vessels. Energy saving and water recycling were critical considerations during engineering.
A significant volume of water during purified water generation is normally wasted due to impurities; however, the design facilitated its collection and treatment to re-direct it for other uses in the other facilities such as R&D. This not only reduces the cost of water, but also conserves water and reduces the amount of wastewater generated.
Furthermore, energy and water requirements were substantially lowered by implementing single-use technologies, due to elimination of extensive cleaning and sterilization between each batch and the chemicals used in the process. Comprehensive environmental life cycle analysis studies have shown that single-use technologies, despite plastic waste generated, have less environmental impact compared to traditional stainless steel, as the majority of environmental impacts relate to energy and water consumption during their use.
Complexity
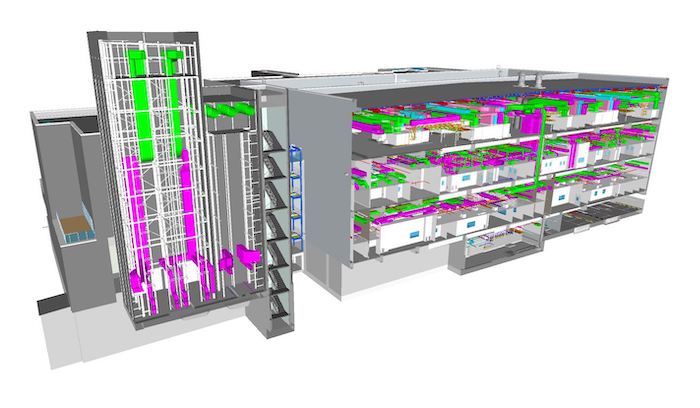
Building information modelling (BIM) is an innovation that transforms traditional project execution. It serves to integrate all the work processes related to the building lifecycle: planning, design, construction, commissioning, operations, and decommissioning. The BIM production system is a digital representation of the physical and technical characteristics of a building and all its systems prior to construction. The virtual model integrates the building geometry and information for the systems that are within it. This permits better coordination in the design phase, better transfer of information to the builders, and permits the owner to improve the management of its infrastructure. By nature, the BIM method involves gathering all relevant information regarding a project in a central database that allows all project participants to exchange information efficiently, significantly improves the coordination at all levels and allows for proactive project management.
By delivering the design in Autodesk RevIT (BIM software), the project benefited from improved coordination and improved conflict detection. There were 12,000 clashes we could identify and resolve, such as by rerouting ducts, which would otherwise only be identified during construction.
In competitive environments, this 3-D design strategy works very well to minimize changes during construction. Minimized change orders is an important key to achieving project objectives and delivering on time and within budget. It helped to better co-ordinate disciplines with ease, maintain accuracy and move projects from design to fabrication to energy-efficient buildings.
Design, visualization, simulation and collaboration using BIM solutions provide greater clarity for all stakeholders across the project lifecycle.
Meeting the owner’s needs
The client’s new facility must be fully compliant with the GMP requirements of various countries such as FDA (U.S.), EMA (Europe), PMDA (Japan) and MFDS (Korea) as they intend to supply their new biopharmaceutical drugs through this facility.
Time to market is essential for our client, and successful completion of design and construction for them to manufacture their clinical trial batches was of critical importance to their business plan.
Quality of construction is another success criteria to our client since their business partner will do pre-inspection once construction has been completed.
SNC-Lavalin received pre-inspection with multiple business partners of the client during the design and construction phase, including from Japan and U.S., and received their full acceptance. Successful pre-inspection is critical for allowing international companies to penetrate the U.S. market. Inspection failures can severely impact and delay projects.
During design, workshops were held both in Canada and Korea which allowed for the SNC-Lavalin Canadian team to meet with the client directly and efficiently. During construction, SNC-Lavalin’s local Korean engineers were present on-site full-time.
SNC-Lavalin has also been commissioned by Aprogen to provide EPCM services for an additional two suites at their existing manufacturing site. The client is further benefiting from the standardized modular design implemented by SNC-Lavalin as part of this project since it can be easily replicated.
Delivering on time and within budget, the successful pre-inspections so far, and additional work from the client to support their further expansion plans, are all indicators of successful project delivery by SNC-Lavalin.
Jinhong (Andrew) Kim is business development and project engineering director for power, grid and industrial solutions (PGIS) infrastructure for SNC-Lavalin Korea. This article originally appeared in the May/June 2021 issue of Canadian Consulting Engineer.